

by Ruby Ghadially, M.D.1 and Gopinathan K. Menon, Ph.D.
Depatment of Dermatology
University of California San Francisco
San Francisco, CA, United States
1Co-Director Epithelial Section of the UCSF Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research

Stem cells have been the rage in life sciences for a while now. This topic has been provoking hope and eliciting moral outrage as well as spawning the birth of new research institutions, bioethical debates, and regulations. This field of science has been honored by a Nobel Prize (awarded to John B. Gurdon and Shinya Yamanaka in 2012) and dishonored by outright scientific fraud.1,2
Love it or hate it, stem cell research cannot be ignored. Today, there are conferences and journals devoted entirely to the topic. The cosmetic industry, striving to keep abreast of scientific advances, has embraced the theme of stem cells—whether it be plant stem cells or logical approaches to impact skin stem cells. This results in an appealing scenario to the marketer and consumer in equal measure.
The fascination and promise of stem cells got a huge shot in the arm when the dream of converting a somatic cell to a pluripotent stem cell became reality (published in Reference 3) and Yamanaka was honored by the Nobel Prize in Medicine in 2012. Induced pluripotent stem (iPS) cells appeared to be the ideal weapon for fighting the age-old (no pun intended) enemy of humanity: old-age. However, even the breakthrough of cloning could not beat aging. Dolly the sheep was cloned (someone did ask: “Why clone sheep when they all look alike anyway?”) with much hope and hype. Alas, it was discovered that Dolly aged pretty fast, as she started life at the biological age of her “parent”.
Now we learn that even iPS cells show signs of the age of the donor from whom the somatic cells originate.4 This is evidenced by the accumulated mutation load and due to epigenetic changes (DNA methylation). Furthermore, paradoxically the mutations were lower when donors were above 90 years of age—a second childhood? This is possibly because in these individuals most of the cell lineages in the blood reach their maximal cell division limits and are replaced by cells that are dividing more slowly, and hence have a lower accumulation of mutations.
So, could stem cells in the aged be persuaded to behave more like young stem cells? With age, stem cells get sluggish—the cellular output from each stem cell is decreased.5 Alterations in the divisional behavior of stem cells may affect this output. Stem cells may self-renew by asymmetric divisions, producing one stem cell and one differentiated cell, or by symmetric divisions producing two stem cells.6 In aged skin there were more asymmetric stem cell divisions—a greater quantity of stem cells seem to be called into action to produce enough cells to maintain the tissue. Could such an increase in stem cell activity be associated with more genetic errors and underly the increased skin cancers in the aged? In many studies, aged stem cells in a young environment perform like young stem cells.7 Can we rejuvenate aged stem cell divisional behavior? Is changing or rejuvenating their environment our best hope in the fight against aging?
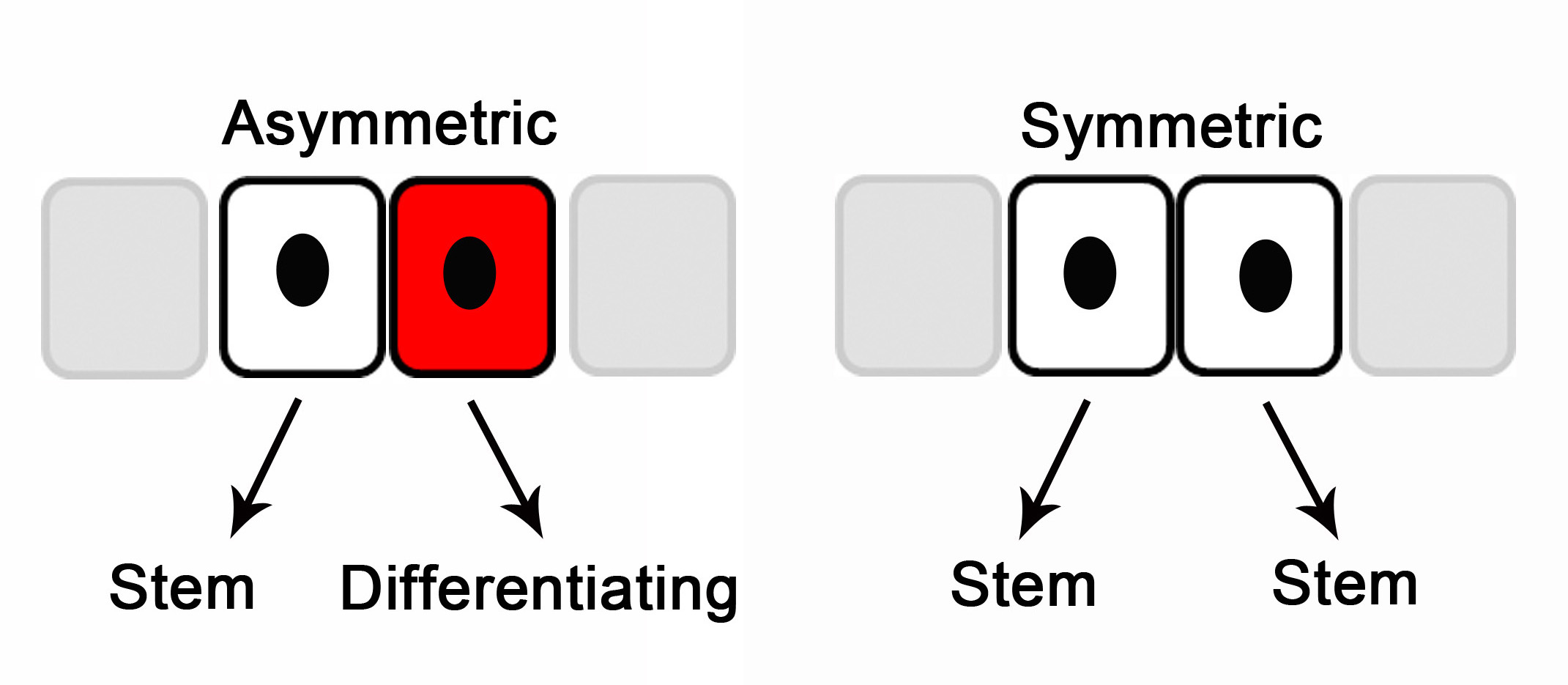
Figure 1: Stem cell self-renewal divisions. Adapted from Reference 8.
References
1. The Nobel Prize in Physiology or Medicine 2012, jointly awarded to John B. Gurdon and Shinya Yamanaka for the discovery that mature cells can be reprogrammed to become pluripotent.
2. H. Obokata, T. Wakayama, Y. Sasai, K. Kojima, M.P. Vacanti, H. Niwa, M. Yamato, and C.A. Vacanti, Stimulus-triggered fate conversion of somatic cells into pluripotency, Nature, 505, 641-647 (2014). Retracted, July 2014.
3. K. Takahashi and S. Yamanaka, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 126, 663-676 (2006).
4. V. Lo Sardo, W. Ferguson, G.A. Erikson, E.J. Topol, K.K. Baldwin, and A. Torkamani, Influence of donor age on induced pluripotent stem cells, Nat. Biotechnol., 35, 69-74 (2017).
5. A. Charruyer, C.O. Barland, L. Yue, H.B. Wessendorf, Y. Lu, H.J. Lawrence, M.L. Mancianti, and R. Ghadially, Transit-amplifying cell frequency and cell cycle kinetics are altered in aged epidermis, J. Invest. Dermatol., 129, 2574-2583 (2009).
6. M. Inaba and Y.M. Yamashita, Asymmetric stem cell division: precision for robustness, Cell Stem Cell, 11, 461-469 (2012).
7. I.M. Conboy, M.J. Conboy, A.J. Wagers, E.R. Girma, I.L. Weissman, and T.A. Rando, Rejuvenation of aged progenitor cells by exposure to a young systemic environment, Nature, 433, 760-764 (2005).
8. A. Charruyer, S. Fong, L. Yue, S.T. Arron, and R. Ghadially, Phycosaccharide AI, a mixture of alginate polysaccharides, increases stem cell proliferation in aged keratinocytes, Exp. Dermatol., 25, 738–740 (2016).