

by Roger L. McMullen
March 15, 2017
In the past two decades significant progress has been made in the development of in vitro-engineered skin. It can be used for patients that require skin grafts, due to loss of skin from injury (e.g., in burn victims), or as in vitro models to study various phenomena in skin. The morphological and biochemical properties of these skin models—known as skin equivalents—have become so advanced that in vitro human epidermal tissues possess many of the same properties of in vivo skin. In two-dimensional cell culture systems aimed at mimicking human skin, cell signaling pathways do not match their in vivo counterpart. On the other hand, the three-dimensional skin equivalent models provide the architecture necessary for such signaling to occur, thereby better facilitating growth and differentiation.1
Skin equivalent models contain a stratified squamous epithelium supported on a collagen framework that is interspersed with fibroblasts. The epithelial differentiation and morphology is similar to that of in vivo human skin. These models are increasingly used in cosmetic science to study the effects of ingredients on the skin, especially for toxicology studies. In fact, they have become increasingly popular in the European Union due to legislation that requires that all safety assessment testing of cosmetic ingredients utilize in vitro models.
In the last several years, the fabrication of skin equivalent models has become increasingly sophisticated.2-5 With the advent of three-dimensional printing technology, researchers in biotechnology are now able to fabricate tissues and organs, which can be used as implants, prosthetic devices, and anatomical models.6 Modern approaches to skin tissue engineering rely on starting with a temporary scaffold (e.g., a hydrogel) to which cellular components of skin are distributed using three-dimensional printing. Cells—keratinocytes and fibroblasts—attach to the scaffold and then proliferate as well as fabricate the extracellular matrix. For illustration, see Figure 1.
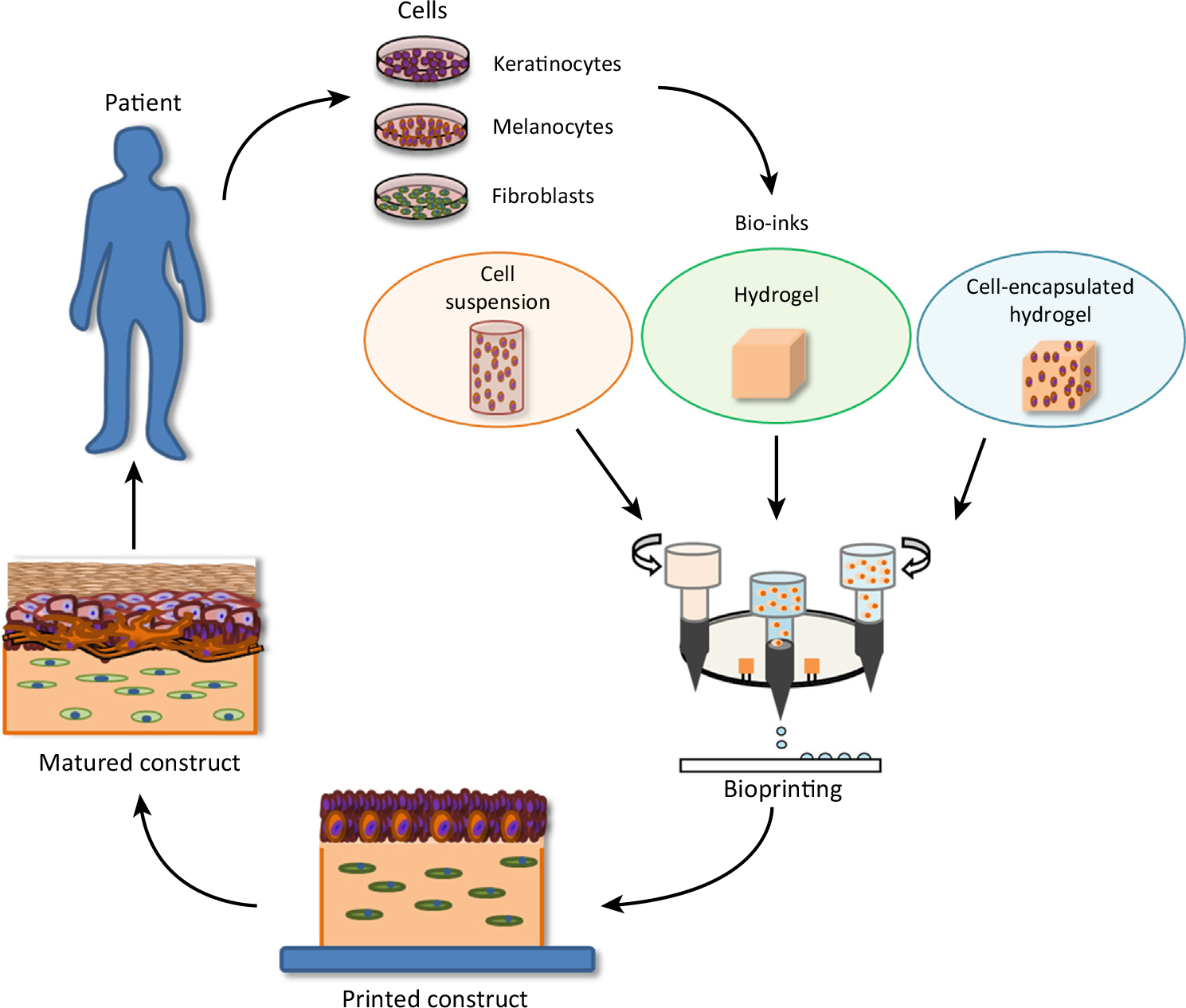
Figure 1: Three-dimensional bioprinting of skin. Reprinted from W.L. Ng et al., Skin bioprinting: impending reality or fantasy? Trends Biotech., 34, 689-699, copyright (2016), with permission from Elsevier.
Bioprinting methods consist of extrusion, inkjet, laser, and microvalve-based systems. Overall, the laser- and microvalve-based approaches have been the most successful in terms of skin tissue engineering.5 In most three-dimensional printing applications, materials are deposited in a two-dimensional array and subsequent layers are printed along the z-axis to generate the three-dimensional object. Some of the notable issues with skin bioprinting consist of problems with pigmentation (melanin), vascularization, and hair follicles as well as the difficulty in obtaining a fully differentiated epidermis.7 Therefore, it is challenging to obtain barrier function properties similar to that of in vivo skin. In any event, tissue engineering methods that rely on bioprinting of skin hold much promise in the future to obtain functional skin grafts that can be used in the clinic and to create accurate models that can be used for safety testing of new ingredients.
References
1. A. Margulis, W. Zhang, and J.A. Garlick, In vitro fabrication of engineered human skin, Methods in Molecular Biology, Vol. 289: Epidermal Cells: Methods and Protocols, Ed. K. Turksen, Humana Press: Totowa, NJ (2005).
2. N. Cubo, M. Garcia, J.F. del Cañizo, D. Velasco, and J.L. Jorcano, 3D bioprinting of functional human skin: production and in vivo analysis, Biofabrication, 9, 015006 (2017).
3. L.J. Pourchet, A. Thepot, M. Albouy, E.J. Courtial, A. Boher, L.J. Blum, and C.A. Marquette, Human skin 3D bioprinting using scaffold-free approach, Adv. Healthc. Mater., 6, 1601101 (2017).
4. D. Singh, D. Singh, and S. Han, 3D printing of scaffold for cells delivery: Advances in skin tissue engineering, Polymers, 8, 19, doi:10.3390/polym8010019 (2016).
5. W.L. Ng, S. Wang, W.Y. Yeong, and M.W. Naing, Skin bioprinting: impending reality or fantasy? Trends Biotech., 34, 689-699 (2016).
6. C. Lee Ventola, Medical applications for 3D printing: current and projected uses, P&T, 39, 704-711 (2014).
7. S. Michael , H. Sorg, C.-T. Peck, L. Koch, A. Deiwick, B. Chichkov, P.M. Vogt, and K. Reimers, Tissue engineered skin substitutes created by laser-assisted bioprinting form skin-like structures in the dorsal skin fold chamber in mice, PLoS One, 8(3): e57741. doi: 10.1371/journal.pone.0057741 (2013).