

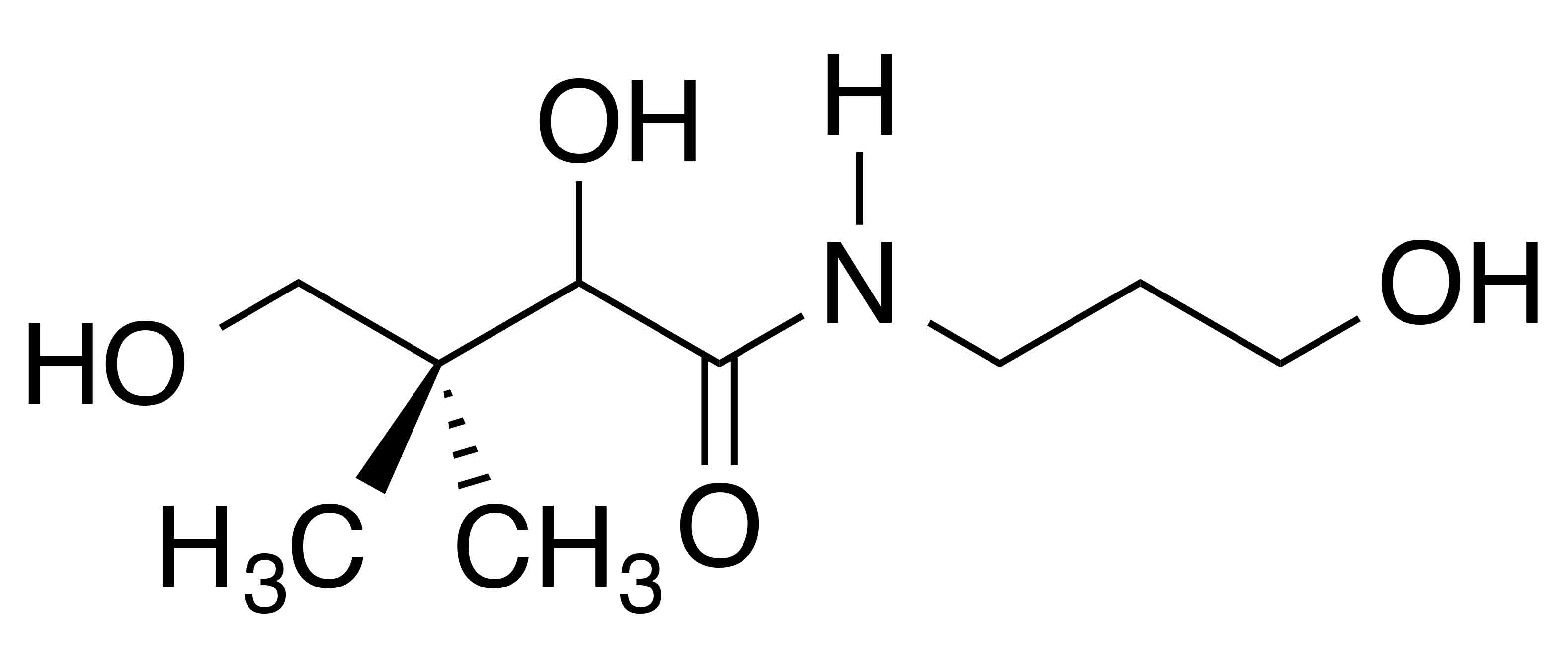
INCI name: panthenol Synonyms: dexpanthenol, DL-panthenol, DL-pantothenol, DL-pantothenyl alcohol
Molecular formula: C9H19NO4
Molecular weight: 205.25 g/mol
IUPAC name: (2R)-2,4-dihydroxy-N-(3-hydroxypropyl)-3,3-dimethylbutanamide
CAS number: 16485-10-2; 81-13-0
EC number: 240-540-6
Panthenol, also known as provitamin B5, is the biologically active precursor of vitamin B5 (pantothenic acid). A major physiological function of vitamin B5 is its conversion to acetyl coenzyme A (acetyl CoA), which participates in many metabolic processes involving carbohydrates, lipids, and proteins. Panthenol is essentially the alcohol form of vitamin B5.
Panthenol is most well known for its humectant properties—it is hygroscopic—thereby leading to its inclusion in moisturizing formulations. Several reports in the literature highlight panthenol’s moisturizing properties and ability to improve the appearance and physical properties (e.g., smoothness) of skin.1-4 It has a long history of use in cosmetic products and good toxicological profile.5
In addition, topically applied panthenol helps to heal wounds, burns, and dermatitis.6 There have been reports purporting the anti-inflammatory activity of panthenol, which would explain its efficacy as a healing agent.7 In fact, a clinical study demonstrated the anti-inflammatory efficacy of nanotopes loaded with panthenol in the treatment of erythema induced by UV radiation.8 Moreover, it was shown to alleviate inflammation caused by sodium lauryl sulfate treatment.9 A recent study demonstrated that gene expression is modulated as a result of panthenol treatment during wound healing.10
Panthenol is also used in hair care compositions—mostly in conditioner and shampoo formulations—for its humectant properties. A search of the patent literature reveals a number of inventions in which panthenol is used in combination with other ingredients as an adjuvant treatment in hair care. However, there are no comprehensive reports in the scientific literature examining the benefits of panthenol in the treatment of hair.
The detection of the concentration of panthenol in cosmetic and pharmaceutical formulations is important from a quality control and product stability standpoint. A number of studies have focused on chromatography technicques, alone or in combination with mass spectrometry.11-13 Other methods take advantage of the optical properties of panthenol and employ colorimetric or spectrofluoremetric determination of the chromophore.14,15
Properties
Boiling point: 118-120 °C (0.02 mm Hg)16
Solubility: soluble in water, ethanol, methanol, and propylene glycol; slightly soluble in glycerin.16
Density: 1.2 (specific gravity at 20 °C referenced to H2O at 20 °C)16
Refractive index: 1.497 at 20 °C/D16
References
1. F.B. Camargo, L.R. Gaspar, and P.M. Maia Campos, Skin moisturizing effects of panthenol-based formulations, J. Cosmet. Sci., 62, 361-369 (2011).
2. A. Georgalas, Anti-aging actives, Cosmet. & Toil., 128(5), 306-312 (2013).
3. O. Schmidt-Eckey, S. Bielfeldt, M. Brandt, and K.-P. Wilhelm, Skin smoothing and moisturizing properties of a panthenol jojoba spray, SÖFW J., 139(1/2), 20-24 (2013).
4. Surjanto, J. Reveny, J. Tanuwijaya, A. Tias, and Calson, Comparison of anti-aging effect between vitamin B3 and provitamin B5 using skin analyzer, Int. J. PharmTech Res., 9, 99-104 (2016).
5. K.H. Beyer, W.F. Bergfeld, W.O. Berndt, W.W. Carlton, D.K. Hoffmann, A.L. Schroeter, and R.C. Shank, Final report on the safety assessment of panthenol and pantothenic acid, J. Am. College Toxicol., 6, 139-62 (1987).
6. F. Ebner, A. Heller, F. Rippke, and I. Tausch, Topical use of dexpanthenol in skin disorders, Am. J. Clin. Dermatol., 3, 427-433 (2002).
7. A. Kretz, Vitamins in cosmetics, Olaj Szappan Kozmetika, 50(3), 94-96 (2001).
8. W. Baschong, D. Huglin, and J. Roding, D-panthenol loaded Nanotopes providing enhanced anti-inflammatory efficacy. A study on human volunteers, SÖFW J., 125(4), 18-20 (1999).
9. E. Proksch and H.P. Nissen, Dexpanthenol enhances skin barrier repair and reduces inflammation after sodium lauryl sulphate-induced irritation, J. Dermatolog. Treat., 13, 173-178 (2002).
10. R. Heise, C. Skazik, Y. Marquardt, K. Czaja, K. Sebastian, P. Kurschat, L. Gan, B. Denecke, S. Ekanayake-Bohlig, K.P. Wilhelm, H.F. Merk, and J.M. Baron, Dexpanthenol modulates gene expression in skin wound healing in vivo, Skin Pharmacol. Physiol., 25, 241-248 (2012).
11. D. Andre, P. Verite, R. Duclos, A.M. Orecchioni, and F. Failly, Determination of alpha-bisabolol and D-panthenol in cosmetic products by gas chromatography, Int. J. Cosmet. Sci., 13, 137-42 (1991).
12. H.-J. Jeong, M.-H. Lee, K.-W. Ro, C.-W. Hur, and J.-W. Kim, Determination of panthenol, cholecalciferol and tocopherol in cosmetic products by gas chromatography-mass spectrometry in SIM mode, Int. J. Cosmet. Sci., 21, 41-50 (1999).
13. S. Khater and C. West, Development and validation of a supercritical fluid chromatography method for the direct determination of enantiomeric purity of provitamin B5 in cosmetic formulations with mass spectrometric detection, J. Pharm. Biomed. Anal., 102, 321-325 (2015).
14. M.A. Shehata, S.M. Tawakkol, and L.E. Abdel Fattah, Colorimetric and fluorimetric methods for determination of panthenol in cosmetic and pharmaceutical formulation, J. Pharm. Biomed. Anal., 27, 729-735 (2002).
15. M.A. Shehata, M.A. Sultan, S.M. Tawakkol, and L.E. Abdel Fattah, Spectrofluorimetric method for determination of panthenol in cosmetic and pharmaceutical formulations, Saudi Pharm. J., 12, 29-34 (2004).
16. M.J. O’Neil, The Merck Index, 15th ed., The Royal Society of Chemistry: Cambridge, UK (2013).