

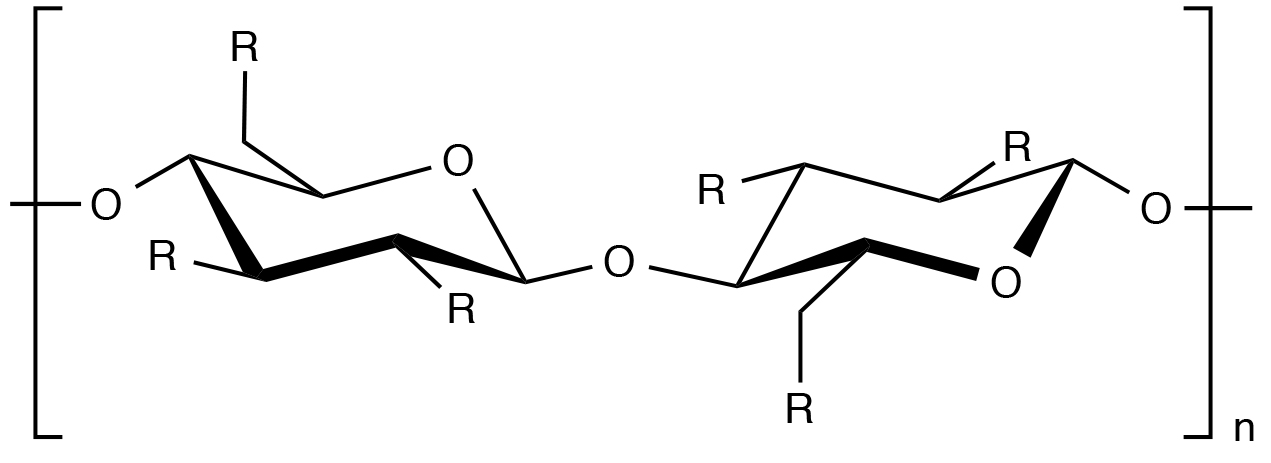
R=OH or R=(OCH2CH)xOH
INCI name: hydroxyethylcellulose
Synonyms: cellulose, 2-hydroxyethyl ether, 2-hydroxyethyl cellulose
Molecular formula: variable
Molecular weight: variable
IUPAC name: 5-[6-[[3,4-dihydroxy-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxymethyl]-3,4-dihydroxy-5-[4-hydroxy-3-(2-hydroxyethoxy)-6-(hydroxymethyl)-5-methoxyoxan-2-yl]oxyoxan-2-yl]oxy-6-(hydroxymethyl)-2-methyloxane-3,4-diol
CAS number: 9004-62-0
EC number: 618-387-5
Hydroxyethylcellulose is derived from cellulose, a polysaccharide that is the most abundant biopolymer in nature and found in the cell walls of plants as well as in wood and cotton. Hydroxyethylcellulose is used extensively in the personal care industry as a thickening agent. It is nonionic (not affected by pH) and soluble in water, thereby providing it with the capacity to thicken the aqueous phase of a formulation. It also has reported uses as a binding agent, emulsion stabilizer, and film former.1
Cellulose is a homopolymer of D-glucose units connected by 1,4-β-glucosidic linkages. The synthesis, or preparation, of hydroxyethylcellulose consists of treating cellulose with alkali followed by reaction with ethylene oxide. As a result, the pendant hydroxyl groups are substituted with hydroxyethyl substituents to a degree that depends on the extent of the reaction. As the degree of hydroxethyl substitution along the cellulose backbone increases, the biopolymer becomes more water soluble.
As indicated by the structural formula above, there are a number of posible structures that may evolve during the ethylene oxide substitution of cellulose. For example, ethylene oxide can react at any of the three hydroxyl substituents on each anhydrous cellulose ring. In addition, ethylene oxide may further react with ethylene oxide units that are already attached to the anhydrous cellulose ring. The molar substitution (MS) of hydroxyethylcellulose is the average number of ethylene oxide units attached to the anhydrous cellulose ring while the degree of substitution (DS) is the average number of hydroxyl groups on the ring that have been substituted with ethylene oxide units.2
Properties
Hydroxyethylcellulose is a biopolymer. Therefore, many of its properties will depend on its molecular weight and the degree of substitution of hydroxyethyl or hydroxyl groups on the pyranose ring.
Solubility: water (soluble), alcohol (soluble up to 70 °C), and organic solvents (soluble).3
Surface tension: 64 dynes/cm.3
Refractive index: 1.337.3
References
1. T.E. Gottschalck and J.E. Bailey, International Cosmetic Ingredient Dictionary and Handbook, 12th ed., Personal Care Products Council: Washington D.C. (2008).
2. W.F. Bergfeld et al., Final report of the Cosmetic Ingredient Review Expert Panel, Amended safety assessment of cellulose and related polymers as used in cosmetics, March 23, 2009.
3. Cosmetic Ingredient Review, Final report on the safety assessment of hydroxyethylcellulose, hydroxypropylcellulose, methylcellulose, hydroxypropyl methylcellulose, and cellulose, J. Am. College Toxic., 5, 1-59 (1986).
Suggested Reading
1. S.C. Naik, J.F.T. Pittman, and J.F. Richardson, The rheology of hydroxyethyl cellulose solutions, Trans. Soc. Rheol., 20, 639 (1976).
2. W. Wang, F. Li, J. Yu, P. Navard, and T. Budtova, Influence of substitution on the rheological properties and gelation of hydroxyethyl cellulose solution in NaOH-water solvent, Carbohydr. Polym., 124, 85-89 (2015).
3. A.K. Brewer, Analysis of hydroxyethylcellulose in personal care products, EcoSEC GPC System Application Note, Tosoh Bioscience LLC. http://www.obrnutafaza.hr/pdf/tosoh/aplikacije/Hydroxyethylcellulose-HEC.pdf
4. Y. Tezuka, K. Imai, M. Oshima, and T. Chiba, Determination of substituent distribution in cellulose ethers by means of a 13C nuclear magnetic resonance study on their acetylated derivatives: 3. Hydroxyethylcellulose, Polymer, 30, 2288-2291 (1989).
5. J.F. Kennedy, Z.S. Rivera, L.L. Lloyd, F.P. Warner, and M.P.C. da Silva, Determination of the molecular weight distribution of hydroxyethylcellulose by gel filtration chromatography, Carbohydr. Polym., 26, 31-34 (1995).