

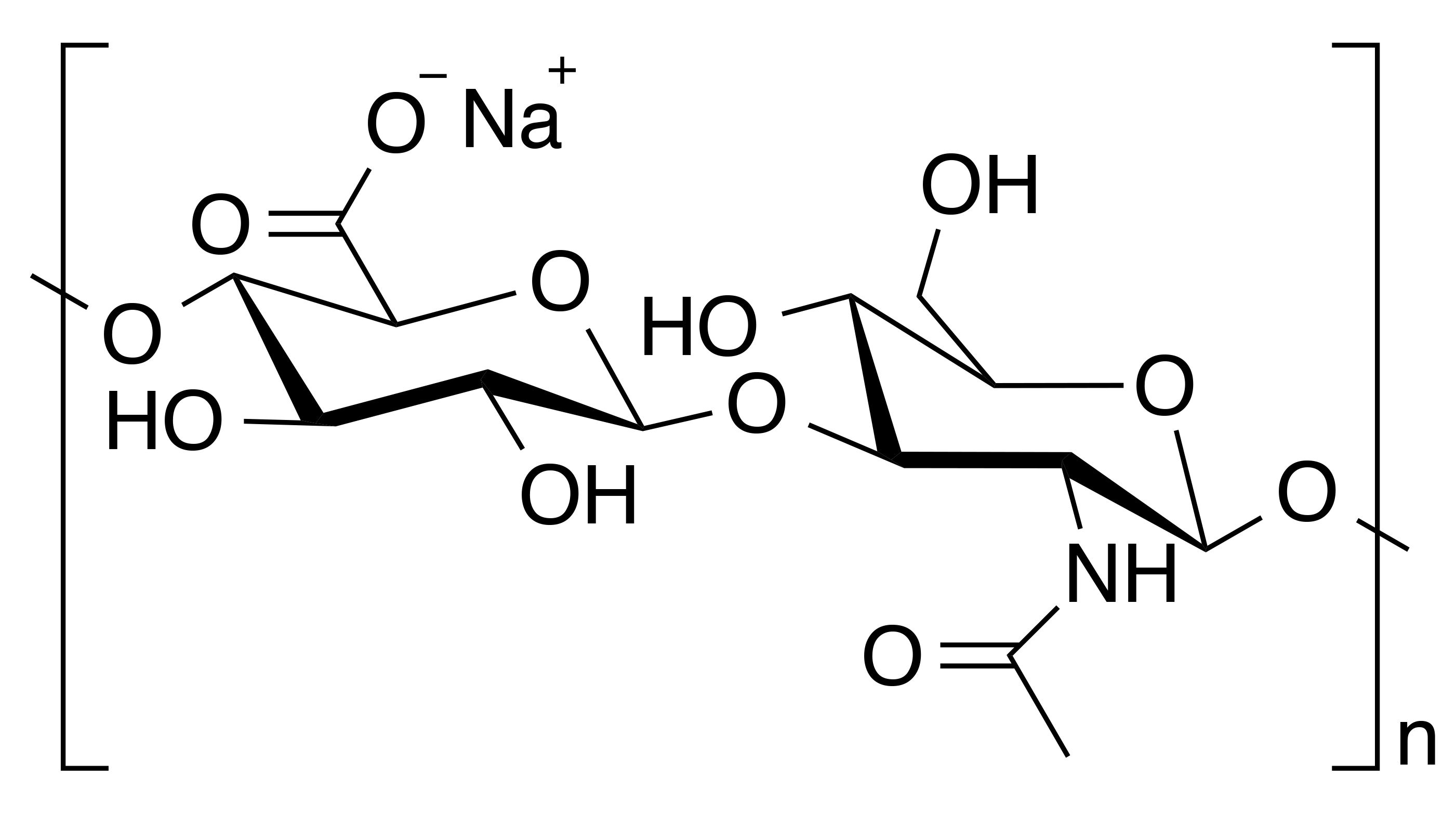
INCI name: sodium hyaluronate
Synonyms: hyaluronan
Molecular formula: variable
Molecular weight: variable
IUPAC name: (2S,3S,4S,5R,6R)-6-[(2S,3R,4R,5S,6R)-3-acetamido-2-[(2S,3S,4R,5R,6R)-6-[(2R,3R,4R,5S,6R)-3-acetamido-2,5-dihydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-2-carboxy-4,5-dihydroxyoxan-3-yl]oxy-5-hydroxy-6-(hydroxymethyl)oxan-4-yl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid
CAS number: 9004-61-9; 9067-32-7 (sodium salt)
EC number: 232-678-0
Hyaluronic acid is a linear polysaccharide of the repeating dissacharide unit consisting of a glucuronic acid and N-acetyl-D-glucosamine moiety that are connected by alternating β 1–3 and β 1–4 glycosidic bonds. It is a glycosaminoglycan, which is found in the extracellular matrix of connective and epithelial tissues. Hyaluronic acid is one of the constituents of the ground substance that embeds the fibrous components and cells of the dermis of skin, although it is also found in the epidermis. In fact, while hyaluronic acid is found throughout the body, the majority of it is found in skin where it is predominantly synthesized by fibroblast cells.1 It should be pointed out that hyaluronic acid is the most abundant glycosaminoglycan found in the dermis. In general, glycosaminoglycans form a fluid matrix by binding with with large quantities of water allowing the dermis to remain hydrated and also sustain physically-induced external pressures resulting in the transformation from a solid to liquid phase.2 In fact, hyaluronic acid can bind up to 1,000 times its weight in water. Typically, it has a a very high molecular mass on the order of millions of daltons.3
In addition to naturally forming an essential part of the dermis structure, hyaluronic acid is also used in skin treatments. Taking advantage of its hydration properties, hyaluronic acid is commonly found in moisturizing formulations. It should be noted that its function depends on climatic conditions. For example, in a dry environment topically applied hyaluronic acid will tend to bind with water already present in the skin, thereby causing dehydration of the skin. In more humid conditions, hyaluronic acid binds with water from the exterior environment and hydrates the skin. In any event, it is typically recommended to formulate hyaluronic acid with occlusive agents, especially in individuals with dry skin.4
In cosmetic dermatology, hyaluronic acid fillers are commonly employed to reduce nasolabial and forehead wrinkles as well as for lip augmentation procedures.5,6 An advantage to the use of hyaluronic acid fillers is their biocompatibility and low potential to cause an immune response. Native hyaluronic acid (linear form of the polysaccharide) only lasts a very short time after injection into skin before it becomes diluted and undergoes enzymatic degradation in the liver. Therefore, hyaluronic acid used in fillers is crosslinked thereby creating a water-insoluble gel that is stable for a period of several months in the skin.5 The degree of crosslinking effects the rheological properties of the gel as well as its swelling behavior.
Hyaluronic acid also has anti-inflammatory and wound healing properties.7 Present in the lower layers of the epidermis, current scientific evidence points to a regulatory and modulatory role by hyaluronic acid in which it binds with proteins and cell surface receptors.8 Until recent years, hyaluronic acid was thought to be just a large hydrophilic polysaccharide of the dermis that absorbs water and contributes the mechanical properties of skin. Now, researchers are starting to unveil a number of important biological functions played by this molecule in skin homeostasis.
References
1. R.M. Kavasi, A. Berdiaki, I. Spyridaki, E. Corsini, A. Tsatsakis, G. Tzanakakis, and D. Nikitovic, HA metabolism in skin homeostasis and inflammatory disease, Food Chem. Toxicol., 101, 128-138 (2017).
2. Z.D. Draelos and P.T. Pugliese, Physiology of the Skin, 3rd ed., Allured Books, Carol Stream, IL (2011).
3. G. Kogan, L. Soltés, R. Stern, and P. Gemeiner, Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications, Biotechnol. Lett., 29, 17-25 (2007).
4. L. Baumann, Cosmeceuticals and Cosmetic Ingredients, Chapter 26, McGraw-Hill: New York (2015).
5. G.D. Monheit and K.M. Coleman, Hyaluronic acid fillers, Dermatol. Ther., 19, 141-150 (2006).
6. A. Tezel and G.H. Fredrickson, The science of hyaluronic acid dermal fillers, J. Cosmet. Laser Ther., 10, 35-42 (2008).
7. R.D. Price, M.G. Berry, and H.A. Navsaria, Hyaluronic acid: the scientific and clinical evidence, J. Plast. Reconstr. Aesthet. Surg., 60, 1110-1119 (2007).
8. E.G. Maytin, Hyaluronan: More than just a wrinkle filler, Glycobiology, 26, 553-559 (2016).