

INCI name: cyclodextrin
Synonyms: cycloheptaamylose, cyclomaltoheptaose
Molecular formula: (C6H10O5)7
Molecular weight: 1134.99 g/mol
IUPAC name: cycloheptapentylose
CAS number: 7585-39-9
EC number: 231-493-2
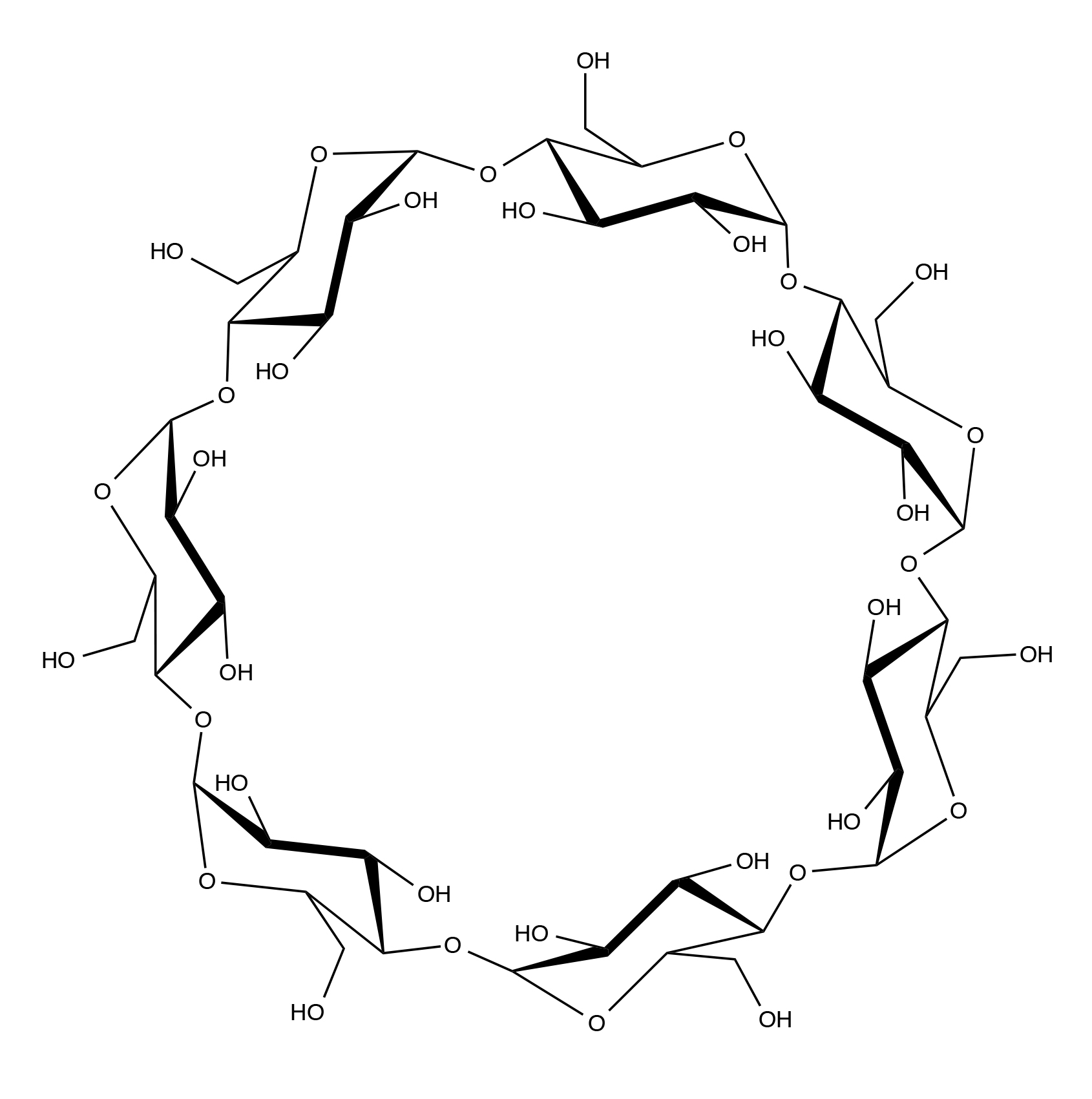
Beta-cyclodextrin is a cyclic oligosaccharide in which seven D-glucopyranose units are bound together by alpha-1,4 linkages. It belongs to the cyclodextrin class of molecules, which differ from each other in terms of the number of glucopyranose units that comprise the ring structure (alpha=6, beta=7, and gamma=8). The cyclodextrins are formed from starch by the enzymes, cyclodextrin glycosyltransferase and alpha-amylase.
Beta-cyclodextrin is commonly employed in many applications due to its lower cost and availability, as compared to the other cyclodextrins.1 It has a toroidal-like structure with an inner hydrophobic cavity, which allows it to be employed as a carrier for a number of different molecules. The cyclodextrin core helps protect the guest molecule from oxidation and decomposition (e.g., from light or heat) and also can increase the guest molecule’s solubility in water, since the outer surface of cyclodextrin is hydrophilic.2 As a result, there are many applications for cyclodextrins in the pharmaceutical, cosmetic, and food industries.3
One specific application area of cyclodextrins in the cosmetic arena includes the encapsulation of essential oils and fragrances.4,5 Generally, fragrance compounds have poor water solubility, making them incompatible in many types of formulations, in addition to often being volatile and instable. Their incorporation into cyclodextrins circumvents many of these issues. Another application is the efficient delivery of antioxidants, which are sometimes unstable, especially in the presence of heat and UV radiation.6
A derivative of beta-cyclodextrin has been successfuly employed to enhance the stability of sunscreen actives.7 Another interesting application of beta-cyclodextrin in the sunscreen landscape is to serve as a carrier for sunscreen actives and to prevent their penetration into the deeper layers of the skin.8 Paradoxically, cyclodextrins have a long history of use as transdermal delivery agents of active molecules.9-11
It should be noted, that of the three natural cyclodextrins, beta-cyclodextrin is the least soluble in water. For this reason, a number of cyclodextrin derivatives have been invented to improve water solubility.12
Properties
Cavity diameter: 6.0 – 6.5 Å1
Melting point: 290-300 °C13
Optical activity: [α]20/D + 162 ± 3°, c = 1.5% in H2O13
Solubility: 1 g/100 mL1
References
1. A. Cutrignelli, A. Lopedota, V. Laquintana, M. Franco, and N. Denora, “Making cosmetics” using cyclodextrins: a review on the application and versatility of cyclodextrins in cosmetic products, H&PC Today, 10(6), 49-51 (2015).
2. H.-J. Buschmann and E. Schollmeyer, Applications of cyclodextrins in cosmetic products: a review, J. Cosmet. Sci., 53, 185-191 (2002).
3. E. Bilensov, Cyclodextrins in Pharmaceutics, Cosmetics, and Biomedicine: Current and Future Industrial Applications, John Wiley & Sons: Hoboken, NJ, 2011.
4. U. Numanoğlu, T. Şen, N. Tarimci, M. Kartal, O.M.Y. Koo, and H. Önyüksel, Use of cyclodextrins as a cosmetic delivery system for fagrance materials: linalool and benzyl acetate, AAPS PharmSciTech, 8(4), Article 85 (2007).
5. G. Wadhwa, S. Kumar, L. Chhabra, S. Mahant, and R. Rao, Essential oil-cyclodextrin complexes: an updated review, J. Incl. Phenom. Macrocycl. Chem., 89, 39–58 (2017).
6. M. Centini, M. Maggiore, M. Casolaro, M. Andreassi, R.M. Facino, and C. Anselmi, Cyclodextrins as cosmetic delivery systems, J. Incl. Phenom. Macrocycl. Chem., 57, 109-112 (2007).
7. N.A. Al-Rawashdeh, K.S. Al-Sadeh, and M.B. Al-Bitar, Physicochemical study on microencapsulation of hydroxypropyl-beta-cyclodextrin in dermal preparations, Drug Dev. Ind. Pharm., 36, 688-697 (2010).
8. J. Shokri, D. Hasanzadeh, S. Ghanbarzadeh, M. Dizadji-Ilkhchi, and K. Adibkia, The effect of beta-cyclodextrin on percutaneous absorption of commonly used Eusolex® sunscreens, Drug Res. (Stuttg)., 63, 591-596 (2013).
9. T. Loftsson and A.M. Sigurdardóttir, Cyclodextrins as skin penetration enhancers. In: Proceedings of the Eighth International Symposium on Cyclodextrins, Eds. J. Szejtli and L. Szente, Springer: Dordrecht, Netherlands, 1996.
10. T. Loftsson and J.H. Olafsson, Cyclodextrins: new drug delivery systems in dermatology, Int. J. Dermatol., 37, 241-246 (1998).
11. J. Hu, Z. Tao, and S. Cao, Unique structure and property of cyclodextrins and their application in transdermal drug delivery, Methods Find. Exp. Clin. Pharmacol., 31, 449-456 (2009).
12. D. Duchêne, A. Bochot, and T. Loftsson, Cyclodextrins and their use in pharmacy and cosmetology, S.T.P. Pharma Pratiques, 19, 15-27 (2009).
13. www.sigmaaldrich.com.