

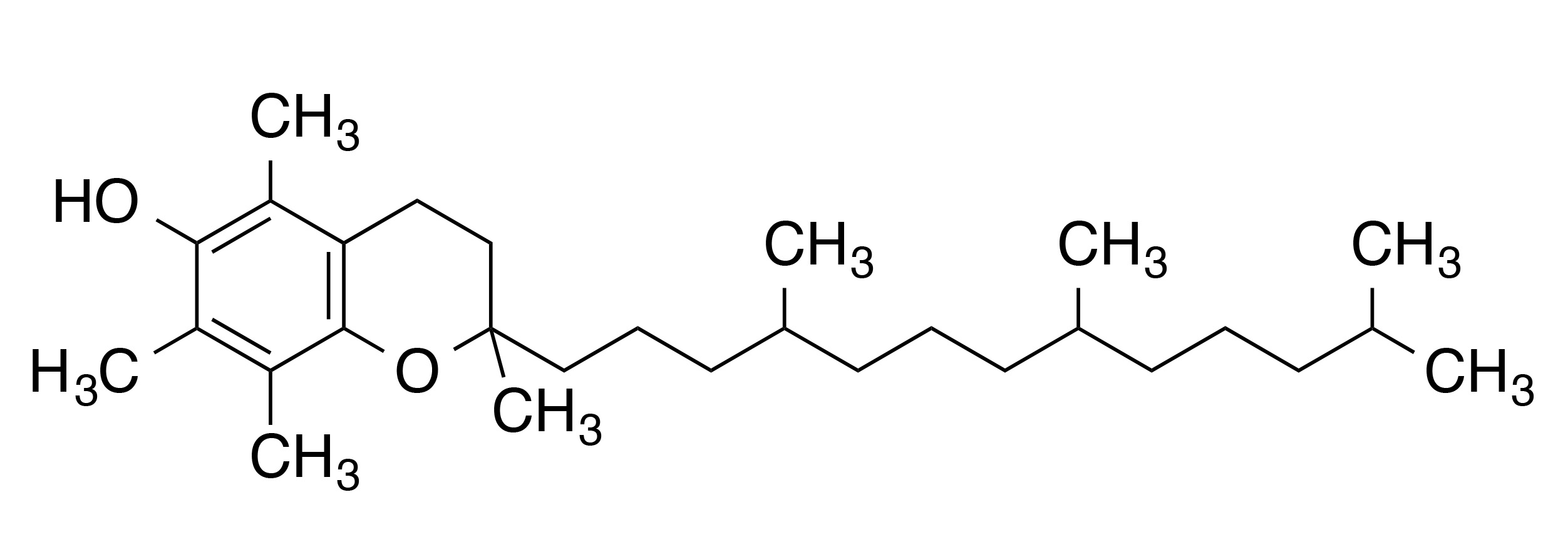
INCI name: tocopherol
Synonyms: vitamin E; D-alpha-tocopherol
Molecular formula: C29H50O2
Molecular weight: 430.71 g/mol
IUPAC name: (2R)-2,5,7,8-tetramethyl-2-[(4R,8R)-4,8,12-trimethyltridecyl]-3,4-dihydrochromen-6-ol
CAS number: 10191-41-0
EC number: 233-466-0
α-Tocopherol is one of the most important naturally occuring antioxidants. It is an endogenous lipid phase antioxidant, which is obtained in the diet (usually from plant oils, nuts, and seeds) and found in human tissues. α-Tocopherol is used extensively in personal care products, especially for the treatment of skin, in which it fortifies the skin’s endogenous antioxidant network. It is found in moisturizers, lotions, psoriasis potions, exfoliants and scrubs, cleansers, acne products, and anti-aging creams. In addition, most contemporary sun care products contain antioxidants (α-tocopherol is the most common), which help ameliorate the detrimental effects of solar radiation through free radical scavenging mechanisms.1
Chemically, α-tocopherol is characterized by its chromanol ring that contains one hydroxyl group and three substituent methyl groups. The hydroxyl group provides this molecule with its antioxidant properties, in which the proton of the hydroxyl group easily reacts with and neutralizes free radical species. α-Tocopherol also contains a long phytyl tail, which is very important for embedding the antioxidant in phospholipid membranes.
There are a number of tocopherol isomers, which differ from α-tocopherol in terms of the substituents around the chromanol ring as well as their stereochemistry. As a simple example, γ-tocopherol contains two methyl, one hydrygen, and one hydroxyl group on the chromanol ring. It should be noted that a common characteristic of all of the tocopherols is the chromanol ring itself and also the long phytyl chain. α-Tocopherol is the most commonly found isomer in tissues. It should also be pointed out that vitamin E refers to all tocopherol and tocotrienol isomers and derivatives.
Properties
Physical: Viscous, pale yellow liquid.2
Boiling point: 200-220 °C (1 atm)2
Melting point: 3 °C3
Specific gravity: 0.95 g/mL (25 °C)3
Solubility: Not soluble in water; soluble in acetone, ethanol, chloroform, ether, and mixtures of ethanol and water;3 also soluble in vegetable oils, mineral oil, and castor oil.
Refractive index: 1.5045 n20/D4
λmax: 298 nm2
Stability: Unstable to UV light, alkalies, and oxidation. Stable to heat (in the absence of O2), strong acids, and visible light.4
References
1. R.L. McMullen, Antioxidants and the Skin, Allured Books: Carol Stream, IL (2013).
2. M.L. Scott, “Vitamin E” in Handbook of Lipid Research: Vol. 2 The Fat-soluble Vitamins, Ed. H.F. DeLuca, Plenum Press: New York, NY (1978).
3. W.M. Haynes, CRC Handbook of Chemistry and Physics, 95th ed., CRC Press: Boca Raton (2015-2016).
4. R.J. Lewis, Sr., Hawley's Condensed Chemical Dictionary, 15th ed., John Wiley & Sons: New York (2007).