

An estimated 57% of the adult population suffers from dentin sensitivity.1 Tooth sensitivity is caused by exposure of dentin, which contains small canals or tubules that are filled with fluid that flow toward the interior of the tooth and excite nervous tissue (see illustration below). Sensitivity results from erosion of the enamel, which is often brought about by excessive brushing and grinding of the teeth that cause eventual wear of the enamel on the crown of the tooth. In addition, gingivitis and receding gums can also cause tooth sensitivity as a result of exposure of dentin at the base of the crown. The condition can be aggravated by eating acidic foods and by drinking hot and cold liquids as well as by using oral hygiene products, such as tooth-whitening toothpaste and mouthrinse, which often contain sensitizing chemicals such hydrogen peroxide and alcohol. Tooth sensitivity also occurs due to cracking of the tooth structure and when there is decay around the edges of fillings. Flora tend to aggregate in these cracks and crevices resulting in the accumulation of acids that damage or weaken the enamel.
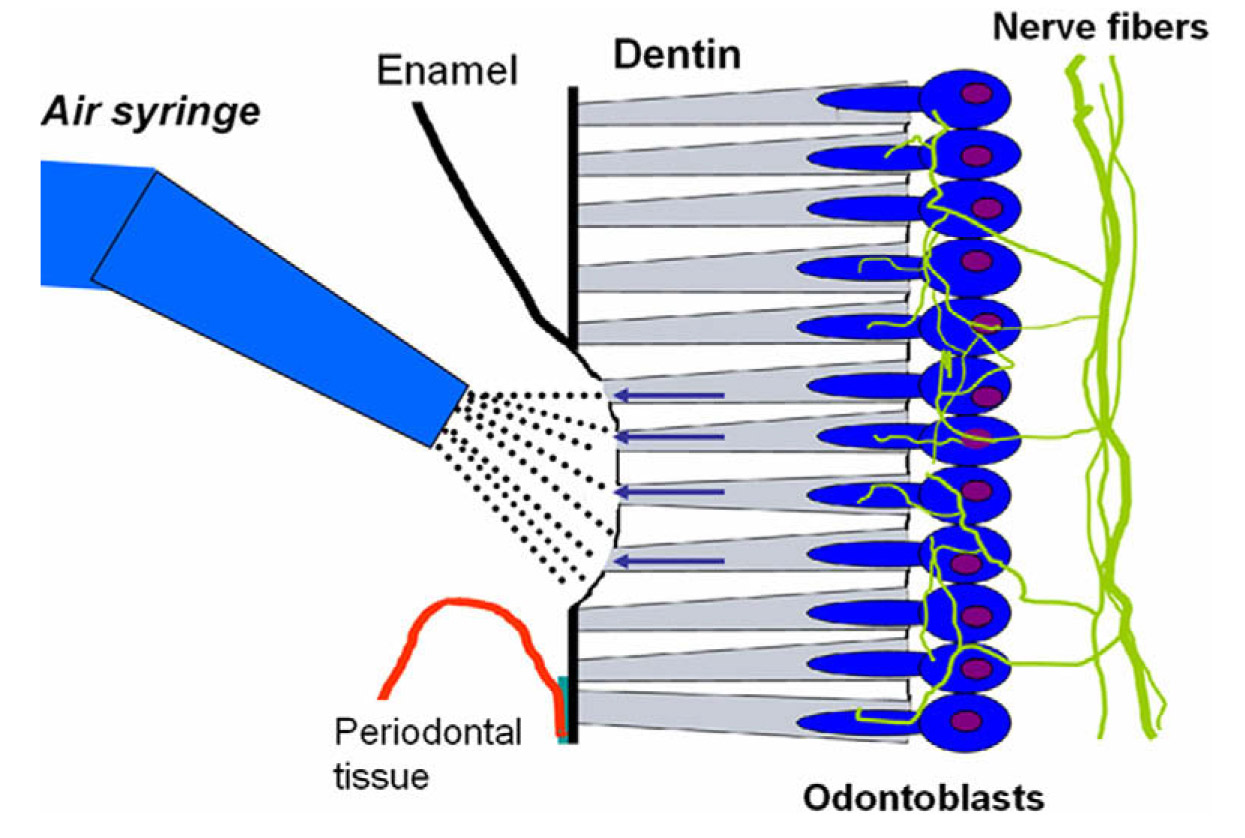
Illustration of the exposure of dentin, which contains small tubules filled with fluid that travels to and excites nerve tissue. Tooth sensitivity occurs as a result of erosion of enamel or receding gums among other factors. Illustration reprinted with permission from K. Markowitz in Reference 2. Copyright (2010), Elsevier.
There are two principle strategies for combating dentin hypersensitivity. One approach is to disrupt the neural response, while the other is to occlude open tubules to disrupt the hydrodynamic mechanism of nerve activation.1 There are several treatment options available for sensitive teeth. Usually the first prescribed mode of action is to employ desensitizing toothpaste. One of the most common active ingredients to include in desensitizing dentifrices is potassium nitrate (KNO3). Evidence suggests that its mechanism of action is through the blocking of nerve cell synapses thereby minimizing nerve excitation and any accompanying pain.3 Typically, potassium nitrate is employed at a concentration of 5% (w/w). Another alternative, known as Pro-Argin technology, incorporates the amino acid arginine in combination with calcium carbonate (CaCO3) in the dentifrice. This technology essentially fills the dentin tubules thereby interfering with the neural response.4 Likewise, stannous fluoride based toothpastes function by a similar mechanism of occlusion of the dentin tubules.5 Most clinical studies designed to determine dentin sensitization are carried out on subjects with existing cases of sensitization. A dental examiner carries out hypersensitivity examinations to determine tactile, thermal, and cold air blast sensitivity, and conducts grading based on a visual analog scale. In vitro tests are also paramount to determine dentin sensitivity as they allow for analysis with more sophisticated instrumentation. For example, the generation of high-resolution images with scanning electron microscopy or confocal laser scanning microscopy allows one to probe the microstructure of the tooth to determine the morphological state of the dentin tubules.
References
1. F. García-Godoy, Dentin hypersensitivity: the effects of an arginine-calcium carbonate and fluoride desensitizing dentifrice. Am. J. Dent., 23(Sp Is A), 2A (2010).
2. K. Markowitz, Pretty painful: Why does tooth bleaching hurt? Med. Hypotheses, 74, 835-840 (2010).
3. P. Walters, Dentinal hypersensitivity: a review. J. Contemp. Dent. Pract., 6(2), 107-117 (2005).
4. D. Cummins, Recent advances in dentin hypersensitivity: clinically proven treatments for instant and last sensisitivity relief. Am. J. Dent., 23(Sp Is A), 3A-13A (2010).
5. D. White, M. Lawless, A. Fatade, A. Baig, R. von Koppenfels, H. Duschner, and H. Götz, Stannous fluoride/sodium hexametaphosphate dentifrice increases dentin resistance to tubule exposure in vitro. J. Clin. Dent., 18(2), 55-59 (2007).