

By M. Julie Thornton and Gillian E. Westgate
Centre for Skin Sciences
Faculty of Life Science
University of Bradford
Bradford, West Yorkshire, UK
October 15, 2017
The phrase 'healthy hair comes from a healthy scalp’ is a common expression, but what is meant by ‘a healthy scalp’? The scalp is the skin that covers the head, and a specific anatomical feature is the large number of terminal hair follicles. The presence of numerous hair canals results in an enlarged epidermal surface, which contributes to a higher level of desquamation.1 The industry focus has mainly been on products designed for cleansing and conditioning the hair and not necessarily for cleansing the scalp, therefore the scalp skin is usually only considered when something goes wrong. So, the question posed is should we consider the scalp as requiring a more specialized approach? The scalp exists in a unique environment. In evolutionary terms, the development of longer hair on the head was in response to the changing environment of our early ancestors and dense curly hair evolved to maintain a sweat-dependent cooling system to protect the brain from the equatorial sun. In this regard, the retention of dense terminal hair on the scalp is presumably a remnant of this protective function. However, the scalp is more complex, and in this article we will consider the scalp microenvironment, its constituent parts, and the factors that are important for maintaining scalp in a healthy state.
Scalp—A Unique Integrated Microenvironment
Scalp skin comprises an epidermis, pilosebaceous units from which terminal hairs grow and associated sebaceous glands secrete sebum, sweat glands, and several different sensory systems in addition to the underlying dermal and adipose (fat) layers (Figure 1). The integrated nature of the scalp skin must factor several physiological processes which are controlled homeostatically (internally) and environmentally (from the outside). It is already known that the outer layers of the skin can act as a biosensor to the environment, changing with ambient humidity and climate.2-4
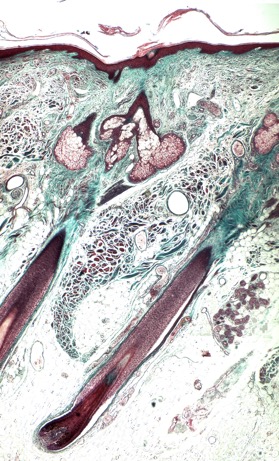
Figure 1: Micrograph of a longitudinal section of human scalp stained with Masson’s trichrome. The epidermis, hair follicles, sebaceous gland, and eccrine sweat glands are stained purple, while the dermis and hair follicle dermal sheath (connective tissue) are blue/green. The bulb of anagen hair follicle, which encompasses the dermal papilla, sits within the hypodermis (adipose) layer. The large sebaceous glands are located towards the upper part of the follicle, whereas the secretory units of the eccrine glands (to the right hand side of the follicle) are found deeper in the reticular dermis.
The scalp, in the areas of terminal hair growth, is a multi-layered structure 5-8 mm from the surface to the base of the dermal fat layer at the level of the terminal hair follicle bulbs. The viable epidermis and situated just above that, the stratum corneum and its surface ecosystem, form the visible scalp, which is a pale, even slightly translucent structure extending between hair follicles and some further into the opening of the hair canals. Caucasian scalp skin is not strongly pigmented, but can respond to excessive UV radiation by burning, tanning, or freckling, especially with thinning hair. The stratum corneum on the scalp is of prime importance, serving as a barrier both to trans-epidermal water loss (TEWL) and preventing entry of toxic materials. A healthy stratum corneum requires the right balance of natural moisturizing factors, epidermal and sebum derived lipids, urea, and lactic acid, combined with normal cell turnover to maintain the correct structure and properties of the barrier.5,6 The scalp stratum corneum, with around 12 layers, is slightly thicker than the face and neck but has fewer layers than the trunk, perhaps reflecting its relative exposure to the environment.7 Factors that affect TEWL and cell turnover include age, environment, hormones, and mild inflammatory conditions.4,5,8-10 A range of useful methods and healthy scalp biomarkers are emerging from such studies (Table 1).6
Table 1. Biomarkers as useful indicators of healthy scalp.
| Biomarker | Source | Role in Scalp Skin |
|---|---|---|
| Natural moisturizing factor (NMF)2 (pyrrolidone carboxylic acid and urocanic acid) | Epidermal filaggrin breakdown products | Moisturizing and maintaining barrier function |
| Lactate2 | Sweat | pH regulation, maintaining the surface pH as acidic |
| Urea2 | Filaggrin and sweat | Moisturizing and water retention properties |
| Inflammatory cytokines8 | Epidermis | Low levels in healthy scalp |
| Histamine11 | Epidermis | Low levels in healthy scalp |
Lipids – ceramides12 |
Epidermis | Vital for structural integrity of the barrier and TEWL |
| Sebum lipids including squalene oxidation as a marker13 | Sebaceous gland | Surface protection, oxidized squalene as an indicator of pollution |
| Corneodesmosome proteins and associated enzyme inhibitors9 | Epidermis | Vital for normal skin turnover and desqammation |
| Cornified envelope proteins | Epidermis | Highly expressed in healthy scalp |
What is the Scalp Microbiome and Should I Care for It?
The scalp surface ecosystem also comprises the resident microflora, which together with the barrier form a protective shield for the skin. The microbiome is defined as the composition of microorganisms in the population as detected using genomic technologies.14 The skin microbiome has been intensively studied, although much less attention has been given to scalp.14-16 The microbial ecosystem interacts both with the skin's defense mechanisms including innate immunity, as well as the external environment; which in the context of this article, includes the many products applied to the scalp when washing and caring for the hair. Thus the microbial ecosystem is in synergy with the host, and balancing this relationship is required for maintaining a healthy scalp, which then links through to healthy hair. It has been shown that oxidative damage in the scalp can affect hair quality.17-18 Intriguingly, although the prevalence of the main symbiotic organisms is highly variable across the body, following disturbance, the same profile is restored to each site confirming the importance of the right microenvironment to healthy skin.14,16 This also applies to the scalp, which has its own unique microenvironment and disruptance to this also changes the microbial community demonstrating these close links.19 Indeed, one effect of UV radiation is the induction of hair follicle micro-inflammation, which has been attributed to the light activation of porphyrins produced by bacteria in the hair canal.20 Topical probiotics are emerging as an area with therapeutic benefit in restoring healthy skin, and the development of products that care for the scalp will also need to maintain the ecosystem and its synergies.
Scalp—Both Sensory and Sensitive
Whilst describing the scalp microenvironment, it is also useful to consider scalp sensation and the concept of sensitive scalp as these can be both separate and related factors in a healthy scalp. Scalp skin contains several types of nerves, many intimately associated with the hair follicle, these more typically being mechano-receptors that register touch and movement of the hair, for example in the wind, or when disturbed by a small insect. However, neuropeptide containing nerves extend well into the epidermis and participate in the transmission of itch and sometimes pain via release of neuroendocrine factors. These are nociceptors and can be triggered by both physical and psychophysical stimuli. Thus, sensation is a normal function of a healthy scalp; touch, heat, cold, and response to a chemical stimulus all drive sensation to a trigger. In the case of itch, the trigger can be psychophysical (thinking about itching can make you itch!). A healthy scalp will be sensorially ‘silent’ without a specific external trigger.21 Deliberate sensory stimulation of scalp skin is well documented in Ayurvedic medicine for whole body relaxation, and there are a range of instruments and devices designed to impart a physically pleasurable stimulation via the scalp, which is known to link to the pleasure centres of the brain.
However, sensitive scalp is more complex and forms a subset of sensitive skin as described by several authors and recently reviewed.22 Can a completely healthy scalp also be a sensitive scalp, and would this drive purchase of the types of product described as ‘for sensitive scalp skin’? There is emerging data to suggest that sensitivity may reflect a decline away from healthy, without obvious visible symptoms. Misery et al. first described the prevalence of sensitive scalp in European populations with a significant number (35-40%) of respondents claiming this condition, and with many individuals it was also associated with other issues of concern such as active hair loss and an intrinsically dry or greasy scalp.23,24 However, it is also likely that a change in status, e.g. from normal to dry, may trigger symptoms. However, although environmental factors such as heat, cold, pollution, humidity, and shampoo trigger sensitivity, it is not known if they cause a decline in scalp health per se.25 Seasonal factors could also be in play here, or hormonal changes with a reduction in sebum lipid levels associated with menopause.26 In terms of scalp skin physiology, factors such as reduced ceramides in the stratum corneum, barrier impairment, and lower levels of hydration predispose the scalp to the ingress of every day ‘irritants’ whether from pollution, hair care products, or metabolites generated locally in the skin.27 Urban living raises the question of whether pollution affects hair and scalp. This has been investigated in studies looking at the most common types of pollution, including UV induced oxidation of proteins and lipids, cigarette smoke, and associated polycyclic aromatic hydrocarbons and particulates: vehicle fumes, ozone, and heavy metals.28,29 Thus the scalp surface ecosystem when maintained in a healthy state is much more likely to be resilient to these external stressors.
So, could a ‘super healthy scalp’ be the goal of effective scalp care which will make it less likely to be sensitive and more resilient to daily life? This is a question now posed by many products and brands. The evolution of new measurement methods and emerging understanding of how the skin, microflora, and environment interact will help technologists understand how to achieve the nirvana of beautiful healthy hair from a super healthy scalp.
References
1. R.M. Trüeb, Shampoos: Ingredients, efficacy and adverse effects, J. Dtsch. Dermatol. Ges., 5, 356-365 (2007).
2.A.V. Rawlings and C.R. Harding, Moisturization and skin barrier function, Dermatol. Ther., 17(Suppl 1), 43-48 (2004).
3. A.Conti, J. Rogers, P. Verdejo, C.R. Harding, and A.V. Rawlings,. Seasonal influences on stratum corneum ceramide 1 fatty acids and the influence of topical essential fatty acids, Int. J. Cosmet. Sci., 18, 1-12 (1996).
4. J. Rogers, C. Harding, A. Mayo, J. Banks, and A. Rawlings, Stratum corneum lipids: the effect of ageing and the seasons, Arch. Dermatol. Res., 288, 765-770 (1996).
5. E.Y. Bonnist, P.D. Pudney, L.A. Weddell, J. Campbell, F.L. Baines, S.E. Paterson, and J.R. Matheson, Understanding the dandruff scalp before and after treatment: an in vivo Raman spectroscopic study, Int. J. Cosmet. Sci., 36, 347-354 (2014).
6. J.R. Schwartz, A.G. Messenger, A. Tosti, G. Todd, M. Hordinsky, R.J. Hay, X. Wang, C. Zachariae, K.M. Kerr, J.P. Henry, R.C. Rust, and M.K. Robinson, A comprehensive pathophysiology of dandruff and seborrheic dermatitis - towards a more precise definition of scalp health, Acta Derm. Venereol., 93, 131-137 (2013).
7. Z. Ya-Xian, T. Suetake, and H. Tagami, Number of cell layers of the stratum corneum in normal skin - relationship to the anatomical location on the body, age, sex and physical parameters, Arch. Dermatol. Res., 291, 555-559 (1999).
8. K. Kerr, T. Darcy, J. Henry, H. Mizoguchi, J.R. Schwartz, S. Morrall, T. Filloon, R. Wimalasena, G. Fadayel, and K.J. Mills, Epidermal changes associated with symptomatic resolution of dandruff: biomarkers of scalp health, Int. J. Dermatol., 50, 102-113 (2011).
9. B. Singh, M. Haftek, and C.R. Harding, Retention of corneodesmosomes and increased expression of protease inhibitors in dandruff, Br. J. Dermatol., 171, 760-770 (2014).
10. P. Florence, C. Cornillon, M.F. D'Arras, F. Flament, S. Panhard, S. Diridollou, and G. Loussouarn, Functional and structural age-related changes in the scalp skin of Caucasian women, Skin Res. Tech., 19, 384-393 (2013).
11. K. Kerr, J.R. Schwartz, T. Filloon, A. Fieno, K. Wehmeyer, J.C. Szepietowski, and K.J. Mills, Scalp stratum corneum histamine levels: novel sampling method reveals association with itch resolution in dandruff/seborrhoeic dermatitis treatment, Acta Derm. Venereol., 91, 404-408 (2011).
12. J. Ishikawa, Y. Shimotoyodome, S. Ito, Y. Miyauchi, T. Fujimura, T. Kitahara, and T. Hase, Variations in the ceramide profile in different seasons and regions of the body contribute to stratum corneum functions, Arch. Dermatol. Res., 305, 151-162 (2013).
13. D.M. Pham, B. Boussouira, D. Moyal, and Q.L. Nguyen, Oxidization of squalene, a human skin lipid: a new and reliable marker of environmental pollution studies, Int. J. Cosmet. Sci., 37,357-365 (2015).
14. B. Dréno, E. Araviiskaia, E. Berardesca, G. Gontijo, M. Sanchez Viera, L.F. Xiang, R. Martin, and T. Bieber, Microbiome in healthy skin, update for dermatologists, J. Eur. Acad. Dermatol. Venereol., 30, 2038-2047 (2016).
15. G.I. Perez Perez, Z. Gao, R. Jourdain, J. Ramirez, F. Gany, C. Clavaud, J. Demaude, L. Breton, and M.J. Blaser, Body site is a more determinant factor than human population diversity in the healthy skin microbiome, PloS One, 11, e0151990 (2016).
16. E.A. Grice, H.H. Kong, S. Conlan, C.B. Deming, J. Davis, A.C. Young, NISC Comparative Sequencing Program, G.G. Bouffard, R.W. Blakesley, P.R. Murray, E.D. Green, M.L. Turner, and J.A. Segre, Topographical and temporal diversity of the human skin microbiome, Science, 324, 1190-1192 (2009).
17. R.D. Sinclair, J.R. Schwartz, H.L. Rocchetta, T.L. Dawson, B.K. Fisher, K. Meinert, and E.A. Wilder, Dandruff and seborrheic dermatitis adversely affect hair quality, Eur. J. Dermatol., 19, 410-411 (2009).
18. J.R. Schwartz, J.P. Henry, K.M. Kerr, H. Mizoguchi, and L. Li, The role of oxidative damage in poor scalp health: ramifications to causality and associated hair growth, Int. J. Cosmet. Sci., 37(Suppl 2), 9-15 (2015).
19. C. Clavaud, R. Jourdain, A. Bar-Hen, M. Tichit, C. Bouchier, F. Pouradier, C. El Rawadi, J. Guillot, F. Ménard-Szczebara, L. Breton, J.P. Latgé, and I. Mouyna, Dandruff is associated with disequilibrium in the proportion of the major bacterial and fungal populations colonizing the scalp, PloS One, 8, e58203 (2013).
20. A. Johnsson, B. Kjeldstad, and T.B. Melo, Fluorescence from pilosebaceous follicles, Arch. Dermatol. Res., 279, 190-193 (1987).
21. G.A. Bin Saif, A. Alajroush, A. McMichael, S.G. Kwatra, Y.H. Chan, F. McGlone, and G. Yosipovitch, Aberrant C nerve fibre function of the healthy scalp, Br. J. Dermatol., 167, 485-489 (2012).
22. L. Misery, K. Loser, and S. Stander, Sensitive skin, J. Eur. Acad. Dermatol. Venereol., 30(Suppl 1), 2-8 (2016).
23. L. Misery, V. Sibaud, M. Ambronati, G. Macy, S. Boussetta, and C. Taieb, Sensitive scalp: does this condition exist? An epidemiological study, Contact Dermatitis, 58, 234-238 (2008).
24. L. Misery, N. Rahhali, M. Ambonati, D. Black, C. Saint-Martory, A.M. Schmitt, and C. Taieb, Evaluation of sensitive scalp severity and symptomatology by using a new score, J. Eur. Acad. Dermatol. Venereol., 25, 1295-1298 (2011).
25. C. Saint-Martory, A.M. Roguedas-Contios, V. Sibaud, A. Degouy, A.M. Schmit,and L. Misery, Sensitive skin is not limited to the face, Br. J. Dermatol., 158, 130-133 (2008).
26. M.Q. Man, S.J. Xin, S.P. Song, S.Y. Cho, X.J. Zhang, C.X. Tu, K.R. Feingold, and P.M. Elias, Variation of skin surface pH, sebum content and stratum corneum hydration with age and gender in a large Chinese population, Skin Pharmacol. Physiol., 22, 190-199 (2009).
27. G. Valacchi, C. Sticozzi, A. Pecorelli, F. Cervellati, C. Cervellati, and E. Maioli, Cutaneous responses to environmental stressors, Ann. New York Acad. Sci., 1271, 75-81 (2012).